Article contributed by Geraint Williams (ALS Global) and Claire Stone (i2 Analytical UK)
Member of the Contaminated Land Working Group
Introduction
Most practitioners understand that field filtration and preservation is considered as a minimum standard when collecting water samples for dissolved metals analysis. However, many people may be unaware of the causes and potential magnitude of negative consequences associated with delayed filtering.
The need for field filtering and preservation
Dissolved metals in water exist in complex equilibria, which can be impacted by many physical and chemical factors, particularly redox conditions, pH or temperature which can trigger changes due to precipitation, co-precipitation, sorption or dissolution of particulate matter. These factors can cause significant positive or negative bias to dissolved metal concentrations. The only way to ensure that water samples collected for dissolved metals will accurately represent conditions at the time of sampling is to conduct filtration in the field immediately after sample collection. The filtered sample should be placed in a dedicated preservative bottle. The type of preservative bottle depends on the specific metal contaminants of concern.
Co-Precipitation of metals
Perhaps the most common pitfall from delayed filtration of dissolved metals is caused by the precipitation of iron oxides and associated co-precipitation of other metals. This happens because ferrous iron is relatively water soluble (up to 100 mg/l), whereas ferric iron is practically insoluble in water under normal environmental conditions (generally <10 µg/l). Ferrous iron exists and is stable in anaerobic waters but when a groundwater sample is exposed to air, oxidation of ferrous iron to ferric iron can occur rapidly, as in Figure 1, sometimes in less than an hour (oxidation rates increase substantially with higher pH).
Figure 1
09:42 10:14 10:29 10:46
Pictures published with the permission of Peter Hewitt, Laing O’Rourke
When iron precipitation occurs in a sample, other metals can co-precipitate, causing substantial changes to the overall dissolved composition of metals in the sample. This is a well-known phenomenon, and precipitation of iron under these circumstances is expected but co-precipitation of other metals is generally less understood.
Case Study on Co-Precipitation
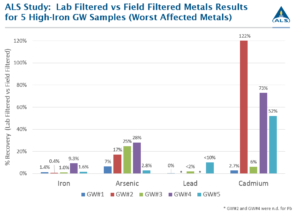
ALS studied the impact of iron precipitation on the co-precipitation of other metals from groundwater samples and found substantial losses of most dissolved metals (in comparison to field filtered metal concentrations), with losses up to 100% in several cases where filtration was delayed (by 6-days in these worst-case examples). Arsenic, lead and cadmium were particularly impacted by co-precipitation, in addition to the expected loss of iron. A summary of the results for arsenic, lead, cadmium and iron are shown in Figure 2.
For these five samples, arsenic losses averaged over 80%, lead losses averaged over 95% and cadmium losses varied significantly by sample, ranging from zero to 97% loss. The impact on sample results can be clearly seen, with many of the samples potentially having key metal concentrations underestimated by a factor of 10 to 100 times if correct filtration techniques were not employed.
Figure 2
Conclusion
There has long been an awareness of the negative impacts of delayed filtering and preservation, however, the magnitude of the effects of co-precipitation of a range of key metal contaminants, not just iron, needs to be taken into account when reviewing results from a laboratory.
Care needs to be taken to ensure the filtration is carried out correctly and effectively. It is not uncommon for filters to block due to high amounts of particulates in solution and in such cases the filtration may become impossible, or there may be filter “bleed through” whereby the particulates bypass the filter and end up in the final laboratory sample. Where this happens, the sample is compromised as the sample is not fully filtered to produce a dissolved sample; it is also additionally compromised in that it is not a “total” metals sample either.
In order to prevent these issues, samples should always be correctly filtered and preserved to ensure they are representative of field conditions and the subsequent analysis of dissolved metals is reliable and valid. Such data is frequently used to inform controlled waters risk assessment or remediation.
References
- AGS Guide to Environmental Sampling, Association of Geotechnical and Geoenvironmental Specialists, 2019
- BS EN ISO 5667-3: 2018 Water quality: Sampling – Part 3: Guidance on the preservation and handling of samples
- BS ISO 5667-11: 2009 Water quality. Sampling. Guidance on sampling groundwaters